
Thyroid Suite
Where thyroid care meets AI-powered breakthroughs
An AI-powered, end-to-end, suite of modular, interoperable applications that are designed to seamlessly integrate into existing thyroid imaging workflows and technology, driving standardization of care, and significantly improving workflow efficiency
Book a demo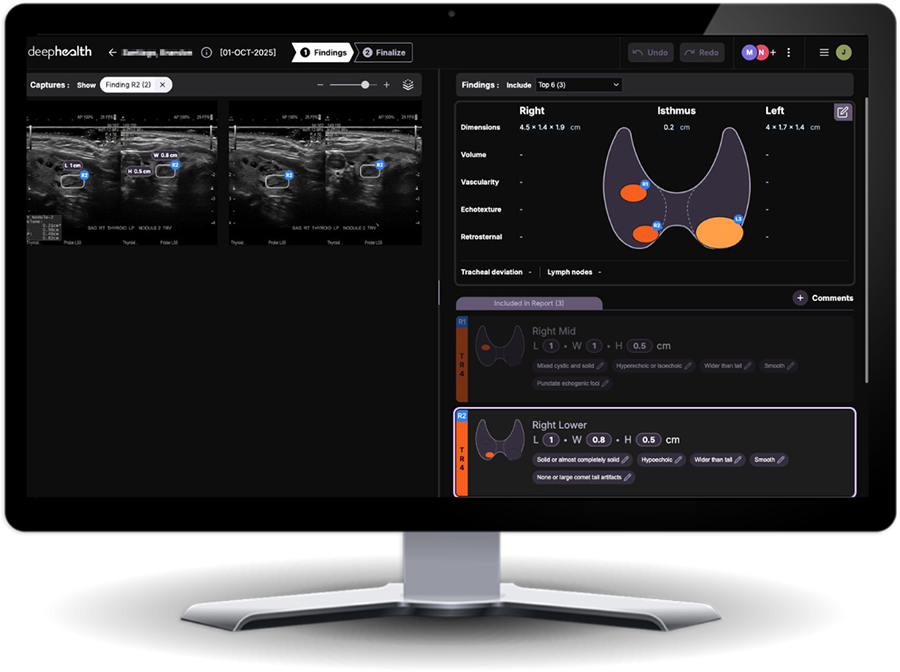

Explore the industry’s most comprehensive integrated portfolio of AI-powered suites of solutions at the European Congress of Radiology.
AI-powered, end-to-end solution for thyroid ultrasound imaging

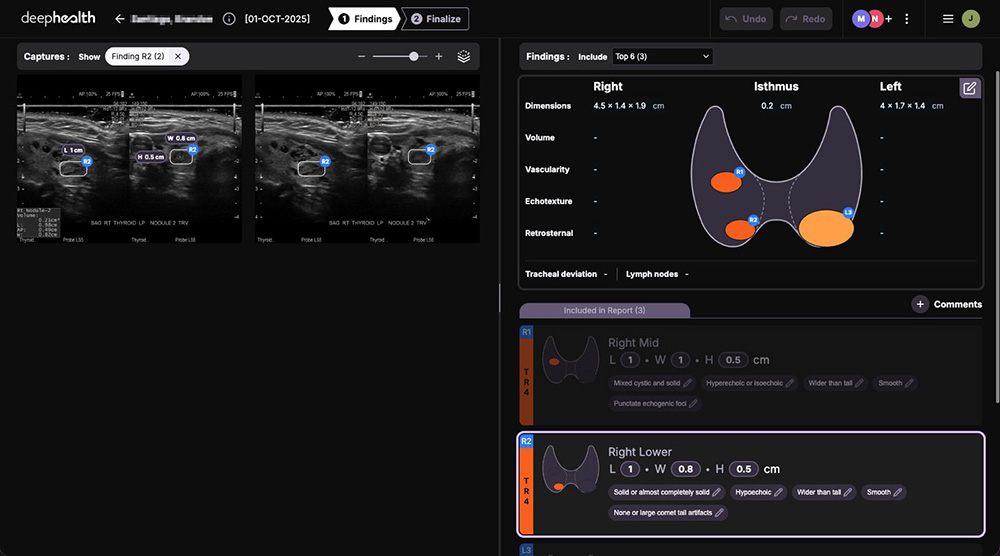
Enables more accurate diagnosis with automated nodule detection & characterization and thyroid sizing according to ACR TI-RADS guidelines
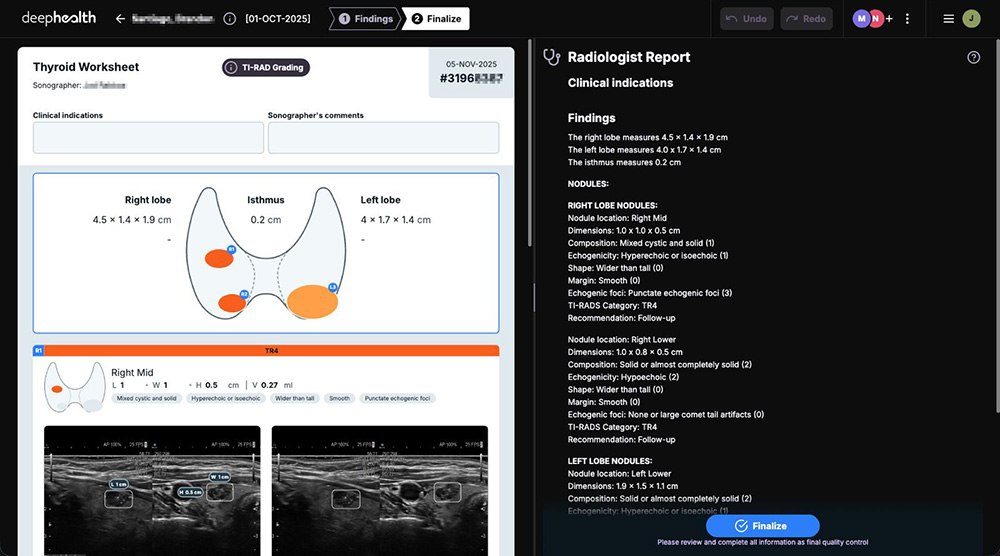
Creates efficient workflows by automatically generating complete, standardized ultrasound worksheets that are sent to PACS or RIS upon finalization.
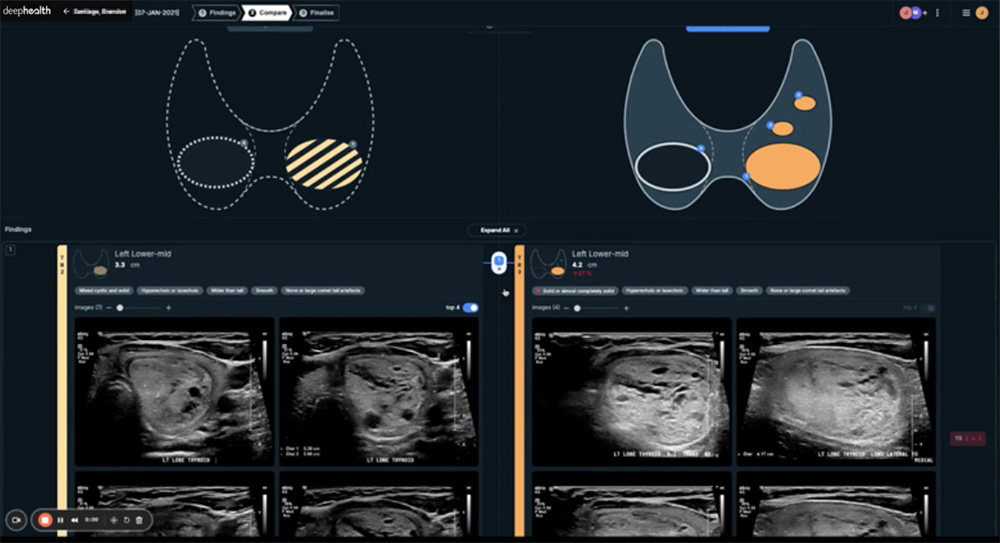
Drives personalized care by comparing cases and presenting changes in nodule size and characteristics for review, enabling better monitoring.
Improves clinical efficiency & consistency with report auto-population and TI-RADS standardization yet allows radiologists to add additional findings in adaptable templates.


Expand patient access and financial sustainability through seamless IT and expanded reimbursement options
Rapid scaling
Leverages cloud-native deployment, supporting cost-effective growth with easy expansion to more applications at your own pace.
Access Anywhere
Enables secure access from any location, limiting IT burden and supporting flexible deployment to different care settings.
Seamless interoperability
Integrates easily with different imaging systems, PACS, RIS, and aligns with customer's clinical protocols.
Financial sustainability
May help deliver higher through put and reduced costs via seamless IT and expanded reimbursement options via new CPT codes.

Proof in practice Evidence, Experience and Resources
Explore Clinical Evidence>90%
of nodules characterized by AI are accepted by radiologists without any changes to their characteristics3
30%
reduction in scan slot time2, allowing more patients scanned per day, without added staff
7%
improvement across 5 categories on average for characterization of TI-RADS descriptors when using AI1
9%
higher level agreement achieved on average across all TI-RADS levels when AI-aided compared to unaided1

Real voices.
Real impact.


Could Healthcare Begin Before Illness Ever Shows Up?
Rather than waiting for patients to experience symptoms, what if care started with prevention, early detection, and access to critical diagnostic tools?
Finding and treating diseases, like cancer, at earlier stages increases survival rates.3 Screening healthy people helps make early detection more possible. By expanding screening for a range of diseases, more people have the change to stay healthy longer- and this shift has the potential to significantly transform population health.
Discover Population Health Solutions[2] Demonstrated up to a 30% reduction in scan slot time. Results from early solution deployment on RadNet sites.
[3] >90% of characterized nodules are accepted by radiologists without any changes to their characteristics. Results are based on data from 240+ RadNet sites and 22,000 thyroid studies [Data on File].
[4] American Cancer Society. Breast Cancer Screening Guidelines, December 2023. Available on the https://www.cancer.org/cancer/types/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html. (Accessed July 16, 2025)
Disclaimers: DeepHealth Thyroid Suite includes DeepHealth Viewer and DeepHealth Thyroid AI. DeepHealth Viewer is Manufactured as eRAD PACS by eRAD and distributed by DeepHealth. DeepHealth Thyroid AI is manufactured as See-Mode Augmented Reporting Tool, Thyroid (SMART-T) by See-Mode and distributed by DeepHealth Inc. Any claims made about Thyroid Suite may reference claims associated with its individual components. Not all products and functionalities are commercially available in all countries.





